This edited article about Robert Wilhelm Eberhard Bunsen originally appeared in Look and Learn issue number 917 published on 18 August 1979.
When a new laboratory was built at Heidelberg, in Germany, in 1855, a chemist was asked to look at the various devices proposed for the supply of heat for chemical experiments. A blowlamp was generally used for these, but the scientist wanted something more convenient to handle.
Finding nothing to suit him, he soon devised a simple means of burning ordinary coal gas with a hot, smokeless flame. His burner was such a good and easy means of obtaining an intense heat that within a short time it became widely used in laboratories. And it remains in use today.
The chemist was Robert Wilhelm Eberhard Bunsen and the device he invented, the bunsen burner, was just one of his many achievements.
The youngest of four sons born to Christian Bunsen, a Prussian diplomat and scholar, Robert was born on 31st March, 1811.
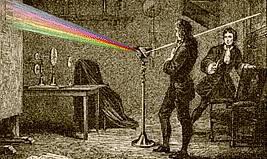
He was educated at Göttingen University, where he studied chemistry, physics, mineralogy, and mathematics. After graduating, Bunsen toured Europe for three years. He visited factories, laboratories and places of geological interest. In October, 1838, he was appointed Professor of Chemistry at Marburg. Subsequently, he spent some time at Breslau, where he met Gustav Kirchoff, with whom he later did important research in spectroscopy.
Appointed to the university of Heidelberg in 1852, Bunsen remained until he retired in 1889 at the age of 78. During this time, as the mastermind at Heidelberg, he made this university one of the great scientific centres of Europe.
Bunsen had begun his first important research at the age of 26, and for six years he had studied compounds of arsenic. In discovering an antidote to arsenical poisoning, he lost the sight of an eye through an explosion. His experiences sapped his enthusiasm for this work, and he left his researches to be carried on by a pupil.
Bunsen had also taken up at the beginning of his career the study of gases given off by blast furnaces. This proved to be of immediate and practical importance. He was able to show that in German furnaces almost half the heat yielded by the fuel was allowed to escape with the waste gases.
Going to Britain to investigate the furnaces, he revolutionised Britain’s methods of producing iron by pointing out that 80 per cent of the heat went up the chimney with the waste fumes, whilst valuable by-products like ammonia were among the gases lost to the atmosphere.
He was able to suggest techniques that could recycle the gases through the furnaces, so using heat that would otherwise be lost. Ways of retrieving valuable escaping materials were also suggested by Bunsen.
Bunsen published his research in his only book Gasometrische Methoden in 1857.
A devoted teacher, Bunsen presented a hundred hours of lectures during each of 74 terms in a course on inorganic chemistry. These kept theoretical aspects to a minimum, for Bunsen was an enthusiast for experimentation. He enjoyed designing apparatus and, being a skilled glassblower, he frequently made his own laboratory glassware. He was also interested in the application of experimental science to industrial problems and he devoted a lot of interest to geology.
In 1841 he ventured into the borderland between chemistry and electricity, as a result of which he invented the carbon-zinc electric cell, also known by his name. By using it to produce an electric arc he obtained, out of a pound of zinc, a light equal to 1,171 candles. Each pound of zinc used in the batteries lasted an hour. Then to measure exactly the candle power of the light he had obtained, Bunsen invented a simple device called a grease-spot photometer.
This uses a white screen with a grease spot at its centre. The sources of light are mounted at opposite sides of the screen. The positions of the lamps are adjusted until the grease spot is no longer distinguishable. The candlepowers can be worked out by squaring the distances of the lamps from the screen.
In 1852 Bunsen began to use his battery in another ingenious way. He passed a current through various solutions, and by so doing separated the constituents. By this means he obtained magnesium in its metallic state and turned his discovery into great significance for photographers by demonstrating the brilliance of magnesium when burnt. He showed how quickly it acted on a photographic plate. Before this, however, he had been able to indulge his interest in geology by accompanying a scientific expedition to Iceland in 1846, the year after the eruption of the volcano Hekla. Sponsored by the Danish government, the expedition lasted nearly four months.
During this time, Bunsen collected gases emitted from the volcanic openings and studied the action of these gases on volcanic rocks. He performed extensive chemical analyses of eruptive rocks, insisting that instead of determining what minerals were in the rock, it was the chemical composition of the rock as a whole which should be ascertained.
Bunsen also explored geysers and made temperature measurements at several depths shortly before one erupted. He found that the temperature of the water in the geyser tube, although high, did not reach boiling point.
He concluded that the driving force for an eruption was supplied by steam that entered the tube under great pressure from volcanic vents at the bottom. As the steam lifted the column of water, the pressure above the water was reduced. This change in the water’s depth results in a lowering of the boiling point and enables the already hot water to boil.
Perhaps it was on this trip that Bunsen gained the idea which enabled him to develop his well-known burner. In analytical chemistry, the burner quickly ousted the blowlamp.
Bunsen also used his burner to identify metals and their salts by their characteristic coloured flames. Other experiments with the burner yielded data for the melting points of metals and rate of evaporation of salts.
In fact, Bunsen was continuously successful as a man of science. In 1868 he worked out methods for separating the metals palladium, ruthenium, iridium, and rhodium that remain in ores after the extraction of platinum, and as part of this project Bunsen constructed a filter pump.
After many other experiments and discoveries he was, in 1842, elected a member of the Chemical Society of London and, in 1882, of the Académie des Sciences of France. The Royal Society honoured him with its Copley medal in 1860 and he received the first Davy Medal in 1877. Finally, Bunsen’s scientific contributions to industry were recognised by Britain’s Society of Arts, which awarded him the Albert Medal in 1898.
On 16th August of the following year, Bunsen died at Heidelberg, which he had helped to make famous and which had provided him with the opportunity for the invention which has earned him immortality.
Another of his achievements was so tremendously important that it dwarfs all others. Following a strange discovery made by a fellow worker in 1860, he took a main part in the work of finding out the significance of the thousands of faint dark lines on the band of colour formed by passing sunlight through a prism. By developing his methods it was possible, in the era before space travel, to get photographs of ion-storms and other masses of flaming elements on the sun.
This success was far more illuminating than his burner flame.






 Posted in:
Posted in: 



